Abstract
Introduction: Hepcidin, a peptide-hormone produced by hepatocytes, is a key regulator of iron homeostasis in mammals. Hepcidin levels are affected by several factors including inflammation, iron concentration, and erythropoietic signaling, and elevated hepcidin levels can lead to anemia. Inflammatory cytokines such as IL-6 induce hepcidin mRNA transcription via JAK/STAT signaling, whereas high serum iron levels and high erythropoietic drive via erythroferrone (ERFE) suppress hepcidin transcription via BMP/SMAD signaling. Anemia is a common problem in patients with myelofibrosis (MF). In a previous study of primary myelofibrosis (PMF) patients, serum hepcidin levels were found to be elevated compared to normal controls and were associated with increased RBC transfusion requirement and reduced survival (Pardanani et al. Am J Hematol 2013;88(4):312-316). Ruxolitinib is a JAK1/2 inhibitor approved for treatment of patients with MF. Treatment with ruxolitinib reduces spleen volume, constitutional symptoms, and inflammatory cytokines, but does not lead to anemia improvement in MF (Verstovsek et al. NEJM 2012;366:799-807). To further assess the relationship between hepcidin and anemia in MF, we measured hepcidin levels in 99 MF patients including 49 PMF and 50 secondary MF (sMF) patients, as well as 24 patients treated with ruxolitinib. We also evaluated the relationship between hepcidin and hemoglobin levels, inflammatory cytokines, and serum iron markers.
Methods: Hepcidin levels (ng/mL) were measured using a commercially available ELISA assay from Intrinsic Lifesciences (Intrinsic Hepcidin IDxTM ELISA) in 99 MF patient plasma samples and 9 normal controls. Plasma cytokine levels (IFN, IL-10, IL-2, IL-2Rα, IL-6, IL-8, MIP-1α, MIP-1β, TNFα, VEGF, TGF-β1, TGF-β2, TGF-β3) were measured via multiplex cytokine analysis (Mesoscale Discovery). CRP, transferrin saturation, and ferritin were measured using standard laboratory methods at our institution. Statistical analyses were performed using GraphPad Prism software.
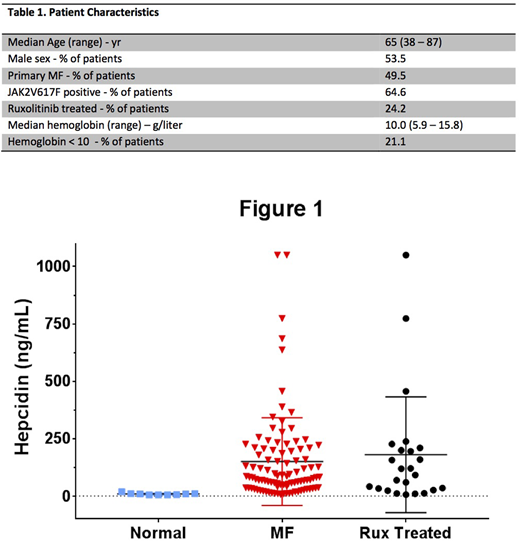
Results: Hepcidin was significantly elevated in MF patients compared to normals (median 81.1 vs 8.5, p <0.001). There was no difference in hepcidin expression between PMF and sMF (median 103.3 vs 80.8, p = 0.380). Hepcidin positively correlated with IL-2Rα and IL-6, but did not correlate with other cytokines evaluated. Hepcidin expression did not differ in samples obtained from patients on treatment with ruxolitinib versus those who were not (median 105.1 vs 80.2, respectively, p = 0.998). A trend between higher hepcidin levels and lower hemoglobin was observed, although it was not statistically significant (p = 0.719). No correlation was found between hepcidin levels and CRP, transferrin saturation, or ferritin. These results are consistent with the previous study confirming that hepcidin is elevated in MF. In addition, we found a correlation between hepcidin and IL-6, which was not observed previously (Pardanani et al. Am J Hematol 2013;88(4):312-316). The lack of significant correlation between hepcidin and hemoglobin may be due to our smaller sample size. Despite ruxolitinib therapy leading to decreases in inflammatory cytokine production, hepcidin levels were markedly elevated in patients treated with ruxolitinib.
Conclusion: These findings indicate that abnormal hepcidin production is a hallmark of both PMF and sMF. Hepcidin expression correlated with IL-6 and IL-2Rα, but not with other inflammatory cytokines, nor with CRP. These observations suggest a complex relationship between inflammation and hepcidin in MF. Although a significant correlation between hepcidin and hemoglobin was not found, this may have been related to the relatively small sample size. In addition, RBC transfusion in a subset of patients may have impacted the lack of correlation between iron indices and hepcidin. Hepcidin was high in patients treated with ruxolitinib, suggesting that suppression of JAK-STAT signaling (such as downstream of IL-6) via ruxolitinib may not be sufficient to normalize hepcidin production. Alternatively, inhibition of hepcidin production via ACVR1/ALK2 has been proposed to be the mechanism behind anemia improvement observed with momelotinib therapy (Asshoff et al. Blood 2017;129(13): 1823 - 1830). Therefore, the role of hepcidin as a therapeutic target for treatment of anemia in patients with MF warrants further exploration.
Zhou:Incyte: Speakers Bureau. Oh:Gilead: Research Funding; Janssen: Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; CTI Biopharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal